Abstract
Introduction:
Acquired hemophilia A (AHA) is a rare disorder characterized by severe, spontaneous bleeding caused by autoantibodies against factor (F)VIII (inhibitors). It is known that onset of AHA is triggered by malignancy, autoimmune disease, dermatological disease, and pregnancy/delivery. As the standard therapy, immunosuppressive therapy (IST) should be started immediately to eliminate inhibitors and hemostatic therapy is also necessary in case of bleeding. Many patients require prolonged bed rest because of the bleeding risk; therefore, it is difficult to determine the best time to start rehabilitation. Additionally, the early deaths and high thrombotic rates are frequently reported in AHA. Since it is a rare disorder, the actual situation has not been fully clarified. This study was to describe the epidemiology and clinical practice of AHA in the real world using a large health claims database in Japan.
Methods:
This was a retrospective observational study using a health claims database provided by Medical Data Vision Co., Ltd. The data period was Apr. 2008-Mar. 2020. Patients who met all of the following criteria were included; patients with disease diagnosis of AHA; patients were hospitalized on the day of AHA diagnosis; and patients had immunosuppressants on/after the date of the first hospitalization. The first date of hospitalization was set as an Index date. Patients with disease diagnosis code of antiphospholipid syndrome, lupus anticoagulant, acquired factor XIII deficiency, acquired von Willebrand disease, or acquired factor V deficiency were excluded. Treatment/procedure patterns (IST, hemostatic therapy, and rehabilitation) and clinical outcome (Activities of Daily Living [ADL], death, and thromboembolism in the hospitalization) in AHA patients were investigated.
Results:
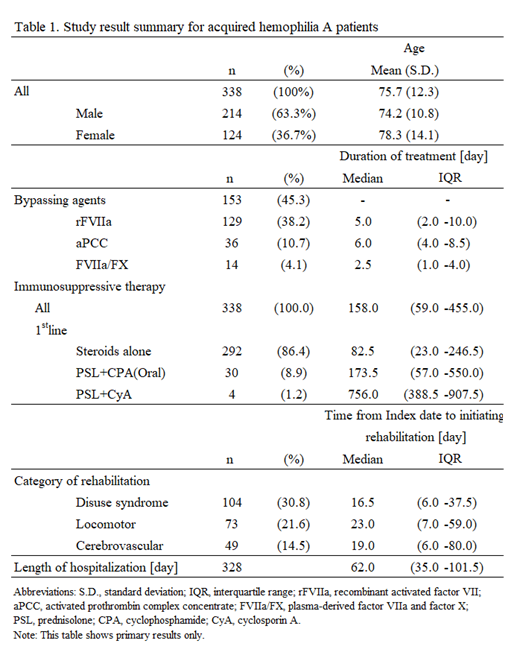
The study population of 338 patients (214 males: 124 females) was with the mean age of 75.7 (21-96) years. A total of 105 patients (pts) (31.1%) had concurrent diseases, including malignancy (61 pts, 18.0%), autoimmune diseases (40 pts, 11.8%), and dermatological diseases (18 pts, 5.3%). In bypassing agent use (153 pts, 45.3%), recombinant activated factor VII (rFVIIa) was the most frequently used (129 pts, 38.2%) followed by activated prothrombin complex concentrate (aPCC) (36 pts, 10.7%), and plasma-derived factor VIIa and factor X (FVIIa/FX) (14 pts, 4.1%). FVIII agent uses (8 pts) were very few. Median duration of treatment for bypassing agents ranged from 2.5 (FVIIa/FX) to 6.0 (aPCC) days. Steroids alone were used predominantly in the first line for immunosuppression (292 pts, 86.4 %) and oral prednisolone was the most frequently used. The category of rehabilitation most commonly implemented in AHA patients was disuse syndrome (104 pts, 30.8%) followed by locomotor (73 pts, 21.6%) and cerebrovascular (49 pts, 14.5%). Median time (days) from Index date to initiating rehabilitation was 16.5 for disuse syndrome, 23.0 for locomotor, 19.0 for cerebrovascular. In the total ADL scores (Barthel Index) in 196 patients with all 10 items, the proportions of patients with less than 70 points were high at both initial admission and final discharge (47.4% and 38.8%, respectively). The median number of times and length of hospitalization were 1.0 time and 62.0 days, respectively. Of evaluable population (328 pts), thromboembolism during hospitalization was recorded in 15 patients, by type of which disseminated intravascular coagulation (10 pts, 3.0%) was the most frequently recorded. Acute coronary syndrome (3 pts, 0.9%), pulmonary embolism and other (1 pt, 0.3%, each) were fewly recorded. The proportion of deaths during hospitalization was 18.6% (63 pts). Table 1 shows study result summary.
Conclusions:
This was the first study in a large AHA population using a health claims database in Japan. From an epidemiological point of view, the number of male patients was slightly larger and the mean age was slightly higher compared to the demographics in previous reports. The possible reason is regional variance or data source, whereas, the treatment patterns and the proportion of deaths during hospitalization are mostly aligned with the previous studies. Also this was the first report publishing the data on ADL and rehabilitation in AHA patients. The results showed that it took median 2-3 weeks to start rehabilitation. Further development of treatment strategies to enable early start of rehabilitation is awaited.
Ogawa: Chugai Pharmaceutical Co., Ltd.: Consultancy. Amano: Chugai Pharmaceutical Co., Ltd.: Consultancy, Speakers Bureau; KM Biologics Co., Ltd.: Research Funding, Speakers Bureau; Bioverativ Inc.: Speakers Bureau; Bayer AG: Speakers Bureau; Shire Plc: Speakers Bureau; Takeda Pharmaceutical Co., Ltd.: Speakers Bureau; Sanofi S.A.: Speakers Bureau; Novo Nordisk A/S: Speakers Bureau; CSL Behring: Speakers Bureau; Pfizer Inc.: Speakers Bureau; Japan Blood Products Organization: Speakers Bureau. Matsuo-Tezuka: Chugai Pharmaceutical Co., Ltd.: Current Employment. Okada: Chugai Pharmaceutical Co., Ltd.: Current Employment. Murakami: Chugai Pharmaceutical Co., Ltd.: Current Employment. Nakamura: Chugai Pharmaceutical Co., Ltd.: Current Employment. Yamaguchi-Suita: Chugai Pharmaceutical Co., Ltd: Current Employment. Nogami: Chugai Pharmaceutical Co., Ltd.: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda Pharmaceutical Co., Ltd.: Honoraria, Research Funding, Speakers Bureau; CSL Behring: Honoraria, Research Funding, Speakers Bureau; Novo Nordisk A/S: Honoraria, Research Funding, Speakers Bureau; Bayer AG: Honoraria, Research Funding, Speakers Bureau; Sanofi S.A.: Honoraria, Research Funding, Speakers Bureau; KM Biologics Co., Ltd.: Honoraria, Research Funding, Speakers Bureau.
Data on the uses of cyclophosphamide, cyclosporin A, and rituximab for acquired hemophilia may be included in the poster presentation. However, with regard to these drugs, treatments for other diseases may also be included because this study was conducted with a secondary use of a health claims database. Off-label drug use is explained in the poster.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal